

Because a hydrogen atom with its one electron in this orbit has the lowest possible energy, this is the ground state (the most stable arrangement of electrons for an element or a compound) for a hydrogen atom. In 1890 Rydberg found that the alkali atoms had a hydrogen-like spectrum that could be fitted by series formulas that are a slight modification of Balmers. at a lower potential energy) when they are near each other than when they are far apart. The hydrogen spectrum had been observed in the infrared (IR), visible, and ultraviolet (UV), and several series of spectral lines had been observed. Electrons when excited by giving sufficient energy will move to higher energy levels. Breaking the symmetry of single-atom catalysts enables an extremely low energy barrier and high stability for large-current-density water splitting.
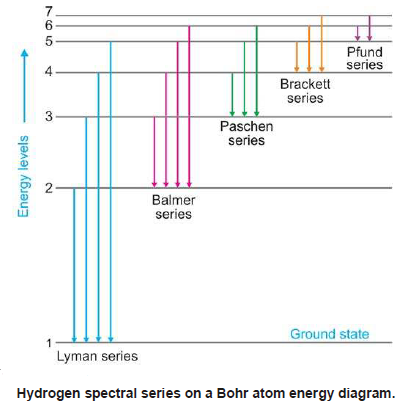
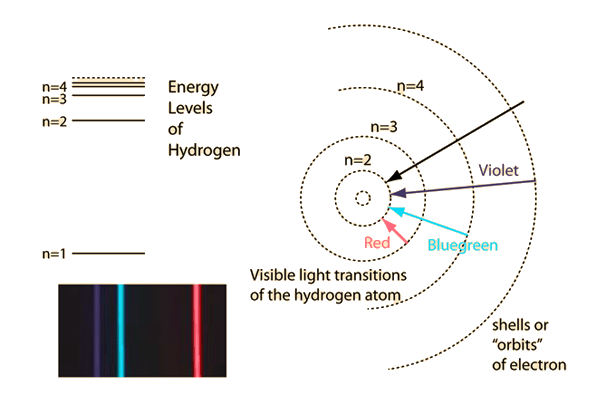
high-resolution XPS spectra of Co 2p, O 1. There is only one electron however it can be excited to multiple. Bohrs theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics. Atomic Ru-modulated Ru-CoO heterostructures as efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. The simplest of all atomic spectra is that of the hydrogen atom. \) indicates that the electron-nucleus pair is more tightly bound (i.e. As you might expect, the simplest atomhydrogen, with its single electronhas a relatively simple spectrum. Hydrogen atomic spectrum are also known as emission spectrum. A hydrogen atom has many spectral lines due to the different transitions that can occur. The emission spectrum of an atom is obtained when excited atoms fall from higher to.


 0 kommentar(er)
0 kommentar(er)
